Assessing Dose Administration of Lu-177 Dotatate Therapy: Quantifying the Residual Dose
Résumé
Au cours d’une radiothérapie, le dotatate marqué au Lu-177 est typiquement dilué dans une poche de sérum physiologique, puis infusé chez le patient par intraveineuse. Une fois administrées, la poche de sérum physiologique et l’intraveineuse sont rincées pour s’assurer que la dose résiduelle soit administrée au patient. Toutefois, changer le sac de perfusion dans une seconde poche de sérum physiologique augmente le risque de contamination. Christian Detlef Raharja voulait savoir si le fait de rincer l’intraveineuse après la radiothérapie au Lu-177 améliore l’administration d’une dose ou si le fait de jeter l’intraveineuse et la poche de sérum physiologique constituerait une meilleure pratique.
Cet article sera présenté en trois segments. Dans cette première partie, l’auteur décrit ses questions et son hypothèse de recherche. Dans le prochain segment, il décrira les procédures et son appréciation.
by Christian Detlef Raharja
With contributions by Edward Sun, Judy Gabrys, and Rebecca Wong
The Michener Institute, University of Toronto
First instalment
When conducting Lu-177 therapy to treat neuroendocrine tumours (NETs), steps are taken to ensure the dose is administered safely to patients. Typically, the Lu-177 dotatate is diluted in a saline bag and then infused through an intravenous (IV) line to the patient. After administration, the saline bag and IV line are flushed to ensure the residual dose is administered to the patient.
However, changing the Lu-177-dotatate-infused bag to the second saline bag increases the risk of contamination. We initiated this quality assurance project to estimate the residual dose of Lu-177 dotatate in the patient’s IV line and determine if flushing is a necessary step.
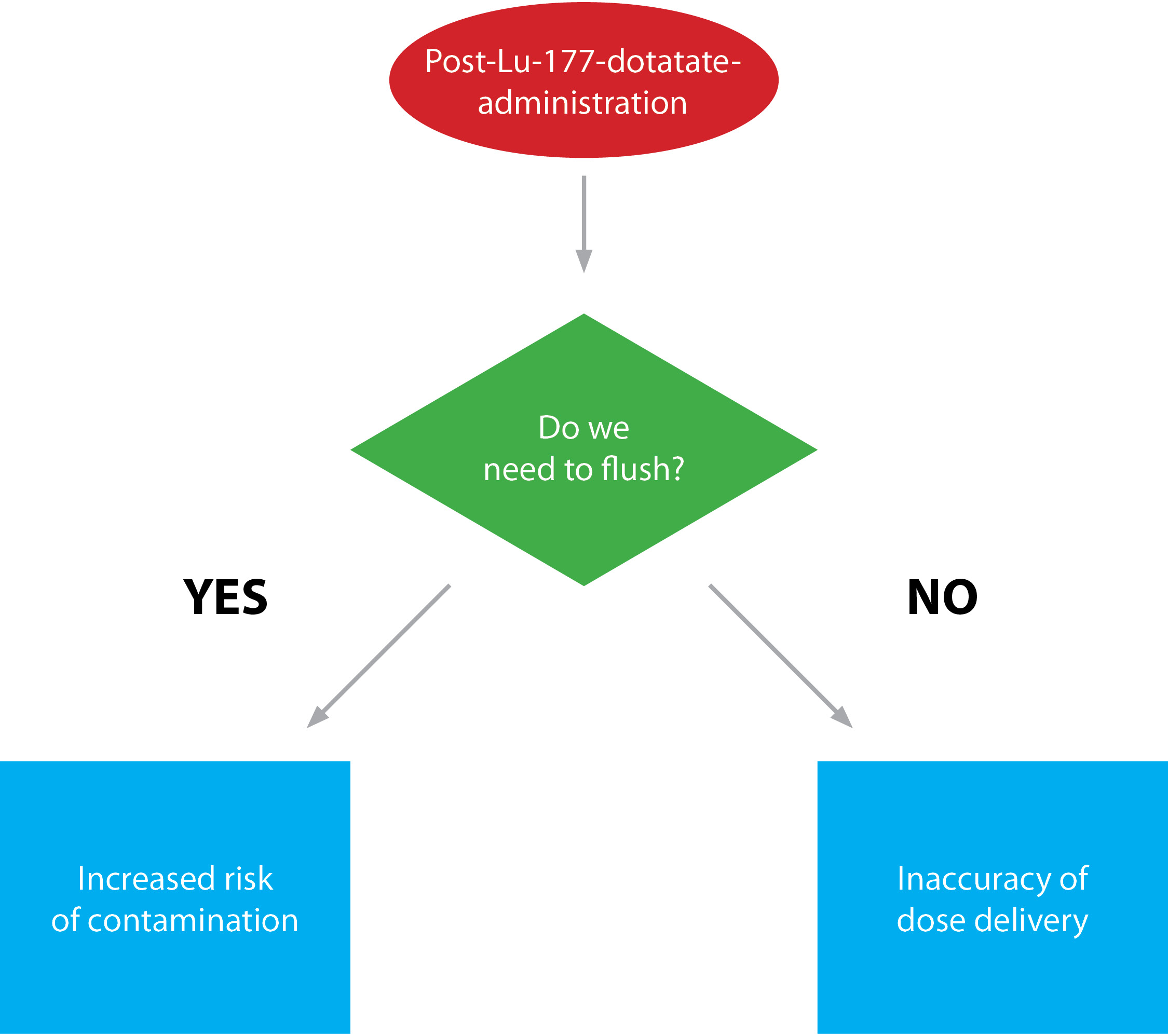
Figure 1: Post-administration of Lu-177 dotatate scenarios
We wanted to know if flushing the line after Lu-177 therapy improved dose administration or if discarding the line and saline bag would lead to better practice (see Figure 1). We did this in two steps:
- We developed a scale to determine the liquid volume in the IV line and chamber and used that scale to measure the activity in the residual dose compared to what was administered.
- We used that measurement to determine if it was necessary to draw a higher dose to compensate for the residual activity (if it was measured to be over 10% of the administered dose).
The results could serve as a guide to technologists looking to develop better practice when administering Lu-177 dotatate. Since this therapy is still a trial in many areas around the world, this study can serve as an important resource when establishing standard protocols for Lu-177 therapy after clinical trials become treatments.
Research question
What is the residual activity in the IV line after initial administration of Lu-177 dotatate?
Hypothesis
Primary – The residual dose within the IV line will not cause a significant impact on the activity administered to the patient (residual activity <10% of the administered dose).
Secondary – Flushing the IV line post-Lu-177-administration will not be a necessary step in the procedure. This would then limit risk of contamination while decreasing the time needed for the therapy.
Background
The field of nuclear medicine allows the visualization of various cancerous sites through imaging after the use of specifically targeting diagnostic radiopharmaceuticals. These sites are also treated with therapeutic radioactive molecules, which are used to kill cancerous cells.[1]
Lu-177 therapy treats metastatic and inoperable NETs through chemical attachment of Lu-177 to the molecules, targeting the over-expressed cellular receptors on the surfaces of the cancerous cells. These cellular receptors are targeted by Lu-177 dotatate specifically, which acts as the Lu-177-labelled somatostatin analogue.[2]
The radioactive decay of Lu-177 passes through tissue by emitting low-energy gamma photons that can be seen through external imaging. Lu-177 decay also emits beta particles where the energy of these particles has been absorbed by the cancerous sites and has killed them.
Lu-177 has a physical half-life of 6.64 days.[3] Due to this long half-life, decay due to transportation would be negligible, which allows for shipment from the reactor production facility to various clinical sites all over the world (Emmett et al., 2017).[4]
Assessing current protocols
For quality assurance in nuclear medicine, we must ensure all protocols are periodically assessed to maintain best practice. Quality assurance sets a benchmark for various procedures,[5] which is an advantage as an educational tool for technologists. Satisfying the requirements for quality would improve the care given to the patients, which is our main goal as health-care practitioners.
This study was conducted at the Princess Margaret Cancer Centre in Toronto, ON. The protocol there is for Lu-177 dotatate to be supplied in volumes ranging from 15 to 30 mL, depending on the patient’s weight in kilograms and the radiation oncologist’s orders. In the radiopharmacy, some saline (equivalent to the patient’s dose) is removed from a 100 mL saline bag and the patient’s dose is added.
The dose is then delivered to the inpatient floor in a radioprotective container. The infusion is set up on the patient’s IV pump and infused at a rate of 200 mL/hr over 30 minutes. After the Lu-177 dotatate infusion, the line is flushed with 25 mL of normal saline over five minutes.
Flushing the line with saline after the Lu-177 infusion, however, increases the risk of contamination to the technologist when the Lu-177-dotatate-infused bag is changed to the 25 mL saline bag. For example, droplets of residual Lu-177 dotatate could splash on the surface of the injection table, the hospital floor, the patient’s gown, or the technologist’s lab coat.
To prevent this, we assayed (tested) the IV line to find out how much residual dose of Lu-177 dotatate remained in the patient’s IV line and determine if flushing is a necessary step in the therapy. Eliminating the flushing step would decrease the risk of contamination and increase workflow efficiency.
Potential for excess radiation exposure false positives
Safely administering Lu-177 dotatate to the patient while limiting contamination is an important aspect of our research. In their article “Contamination, a Major Problem in Nuclear Medicine Imaging,”[6] Kumar et al. assessed a case study that described the effects of radioactive contamination in imaging. A 55-year-old man who was diagnosed with prostate cancer was scheduled for a whole-body bone scan to see if the cancer had spread to his bones. A dose of 740 MBq of 99mTc-methylene diphosphonate was injected intravenously prior to imaging.
The technologists found three abnormal foci of radiotracer near the patient’s right foot. After changing the orientation of the patient from feet first to headfirst, the technologists found the abnormal activity in the head region, which led them to start the acquisition without the patient on the bed. This revealed that the radioactivity was contamination on the collimators.[7]
Kumar et al. describe how contamination can lead to excess radiation exposure to patients and staff, and hinder the workflow of technologists. If the technologists conducting this scan had not identified the contamination in this case, it may have resulted in a false positive and been reported as skeletal metastases. When administering Lu-177 dotatate, contamination on the patient’s clothes may result in a false positive of NETs when imaging later on that day.
Technologist safety
Technologist safety during therapy should not be overlooked when assessing exposure rates. In their paper “External Radiation Exposures from Lutetium-177 Dotatate Therapy Patients,”[8] Erwin et al. aimed to estimate the external, total-body doses and the patient self-attenuated exposure rate to staff involved in Lu-177 dotatate therapy.
The staff’s personal dosimeters were measured during the four-hour infusion period and the uptake period. The study concluded that the dose the nuclear medicine technologist was exposed to was higher than the doses the nurse and electrocardiography technologists were exposed to. The dose per treatment was 2.44 times greater (18.8 +/− 7.2 μSv) than the average daily dose that a nuclear medicine technologist would be exposed to.
Erwin et al. confirmed that there was a significant increase in radiation exposure to staff involved in Lu-177 therapy, especially nuclear medicine technologists. Flushing the IV line increases the risk of contamination, which then increases exposure rates and prolongs the time necessary for the procedure. Eliminating this step could reduce the exposure rate for staff.
Rationale
Ensuring exposure is as low as reasonably achievable (ALARA) when executing nuclear medicine protocols is important. Maintaining best practice for technologists through quality assurance is also an important aspect in ensuring the patient’s safety.
Flushing the IV line following Lu-177 dotatate therapy could increase the risk of contamination if the stopper is not in place or if the line or bag is leaking. An example of contamination is shown in Figure 2, where a small amount of the residual dose spilled on the injection table after flushing the IV line.

Figure 2: Contamination resulted when a small amount of the residual dose (140,000 counts) spilled on the injection table after attempted flushing of Lu-177 dotatate from the IV line.
However, if the IV line and bag are discarded rather than flushed after the administration of Lu-177 dotatate, there would be uncertainty about how much residual activity was left in the line and bag, resulting in uncertainly about the dose administered. If a significant amount of activity is left in the residual dose, the technologist might under-dose the patient. If the residual activity is over 10% of the actual prescribed dose, this would not follow the protocol’s requirements (Wong, 2015).[9] (See Figure 1).
In one case study, a patient was prescribed a reduced Lu-177 dotatate dose due to a significant change in their hemoglobin and neutrophil levels overnight. When the IV line was tested post-administration, 11.5% of the prescribed dose was still in the IV line, which significantly impacts the dose administered. This scenario brought up the uncertainty of the dose remaining in the IV line and initiated this research project.
In their paper “Residual Activities of 99mTc-labelled Radiopharmaceuticals In Routine Nuclear Medicine Practice,”[10] Stavrou et al. examined Tc-labelled radiopharmaceutical administrations to determine the actual amount of activity given to the patient. They found that the mean effective activities were in the range recommended by the European Association of Nuclear Medicine guidelines. The residual activity remaining would thus not compromise the department’s protocols.
Summary
If flushing is not necessary after the administration of Lu-177 dotatate, the risk of contamination could be decreased. In addition, the workflow efficiency may be increased, which would limit the time required for the administration portion of the procedure.
Overall, we wanted a better understanding of the administration of Lu-177 dotatate to ensure the administration portion is as efficient as possible while abiding by the rules of ALARA and the protocol limits of the study. We wanted to answer two questions:
- What amount of the administered dose is remaining within the IV line after administration?
- Is flushing the line after administration of Lu-177 dotatate a necessary step?
In the next instalment, we will describe the study procedures and evaluations.
References
1. Pillai, A., & Knapp, F., 2015. Evolving important role of lutetium-177 for therapeutic nuclear medicine. Current Radiopharmaceuticals, 8(2), 78–85.
2. Kam, B.L., et al., 2012. Lutetium-labelled peptides for therapy of neuroendocrine tumours. European Journal of Nuclear Medicine and Molecular Imaging, 39(S1), 103–112.
3. Kossert, K., et al., 2012. Activity determination and nuclear decay data of 177Lu. Applied Radiation and Isotopes, 70(9), 2215–2221.
4. Emmett, L., et al., 2017. Lutetium 177 PSMA radionuclide therapy for men with prostate cancer: A review of the current literature and discussion of practical aspects of therapy. Journal of Medical Radiation Sciences, 64(1), 52-60.
5. Vinjamuri, S., & Vivian, G., 2010. Quality assurance of reporting in nuclear medicine. Nuclear Medicine Communications, 31(12), 1001–1003.
6. Kumar, N., et al., 2017. Contamination, a major problem in nuclear medicine imaging: How to investigate, handle, and avoid it. Journal of Nuclear Medicine Technology, 45(3), 241–242.
7. CT scanners consist of an X-ray source opposite an arc-shaped array of detectors. The collimators are located immediately in front of the detectors to protect them from scattered X-rays.
8. Erwin, W., et al., 2015. External radiation exposures from lutetium-177 dotatate therapy patients. Journal of Nuclear Medicine, 56(3), 1246.
9. Wong, R., 2015. A prospective phase II, single-arm, multi-centre, study of the efficacy and safety of lutetium-177 octreotate (Lu- dotatate) treatment in patients with somatostatin receptor positive neuroendocrine tumours. Ozmosis Research Inc., 1, 1-56.
10. Stavrou, P.Z., et al., 2016. Residual activities of 99mTc-labelled radiopharmaceuticals in routine nuclear medicine practice. Nuclear Medicine Communications, 37(6), 658–663.
 Christian Detlef Raharja
Christian Detlef Raharja
Christian Raharja is a nuclear medicine student at the Michener Institute, University of Toronto. His current clinical rotation is within the University Health Network, which includes Toronto General Hospital, Toronto Western Hospital, and SickKids Hospital. His work focuses on creating quality assurance projects to improve the current trials and procedures implemented in the hospitals he works in. His hobbies include weightlifting, cooking, paddling (dragon boat), and travelling.
Christian Raharja étudie présentement la médecine nucléaire à l’Institut Michener de l’Université de Toronto. Il effectue présentement son stage clinique au sein du réseau de santé University Health Network, qui inclut le Toronto General Hospital, le Toronto Western Hospital et le SickKids Hospital. Ses recherches sont centrées sur la création de projets d’assurance qualité afin d’améliorer les essais et procédures actuels mis en circuit dans les hôpitaux où elle travaille. L’haltérophilie, la cuisine, le bateau-dragon et les voyages font partie de ses principaux passe-temps.


 Christian Detlef Raharja
Christian Detlef Raharja